Spiration® Valve System for
Allied Professionals
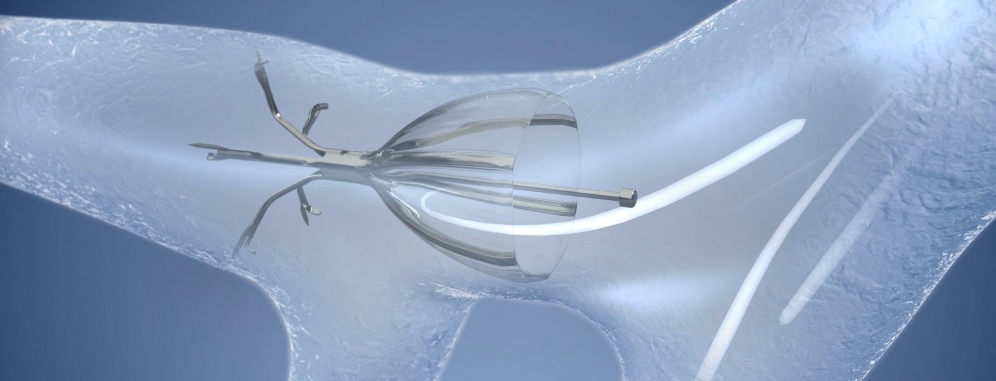
The Spiration Valve System is an innovative endobronchial therapy that offers patients with severe emphysema a customized, minimally invasive treatment option for lung volume reduction with a favorable risk-benefit profile. Patients treated with the Spiration Valve System in clinical trials experienced improvements in breathlessness, lung function, and quality of life.1
COPD & Emphysema
Finding the Right Patient
Emphysema – one of the forms of Chronic Obstructive Pulmonary Disease (COPD) – is a disease that progressives over time and involves the gradual damage of lung tissue, specifically destruction of the alveoli, resulting in trapped air that can cause hyperinflation of the lungs; causing significant breathing challenges. Emphysema symptoms include frequent coughing, wheezing, shortness of breath, tightness in the chest, increased mucus in the lungs, etc.
According to the Centers for Disease Control and Prevention (CDC), 3.5 million people in the United States have been diagnosed with emphysema.2 Smoking is the main cause of emphysema; however, other common causes include air pollutants, alpha-1 antitrypsin deficiency, respiratory infections, etc.

Find a treating physician in your area!
Treatment Center LocatorStages of COPD8
Upon COPD diagnosis, a patient is classified under the following stages based upon their airflow limitation severity:
Group A (GOLD 1):
Were not hospitalized for your symptoms
Group B (GOLD 2):
Have not been hospitalized for your symptoms
Group C (GOLD 3):
Been hospitalized at least once
Group D (GOLD 4):
Been hospitalized more than once
Alpha-1 Antitrypsin Deficiency
The Only Valve Indicated for this Genetic Condition
Alpha-1 antitrypsin deficiency is a genetic condition and can often result in Chronic Obstructive Pulmonary Disease (COPD) and severe emphysema symptoms in patients of any age. The Spiration Valve has been studied and shown to benefit these patients. Spiration Valve placement resulted in improvements in lung function, quality of life, and shortness of breath.1
Potential complications which may be associated with bronchoscopy and/or the Spiration Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, dyspnea and in rare cases, death. Prior to using the Spiration Valve System, please review the full list of prescriptive information at https://svs.olympusamerica.com/prescriptive-information for additional information on indications, contraindications, warnings, precautions and potential complications.
Emphysema Treatment Options
Lung Volume Reduction Surgery (LVRS)
During LVRS, the damaged lung is removed to allow the functioning lung tissue to re-expand. This surgery can help improve gas exchange.Lung Transplant
Most patients with severe, end-stage lung disease can be considered for a lung transplant. The procedure should be considered when someone seems likely to die without the surgery and no other options are available.Bullectomy
In some patients with COPD, bullae can take over large parts of the chest cavity. This causes compression of the surrounding lung tissue. Surgically removing this large sac can be associated with significant benefits and may be the best option for your patient.Endobronchial Valve Treatment
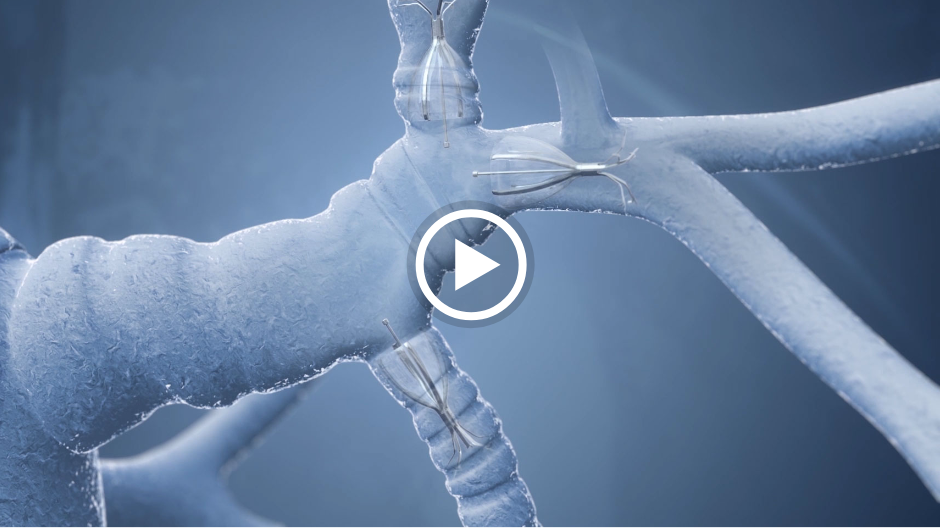
One-way valves can be inserted into a targeted airway of the damaged lung during a minimally-invasive bronchoscopy. The valve will redirect air away from the disease part of the lung towards the healthier parts, allowing patients to breathe easier.
Endobronchial Valve Treatment
A Proven New Direction in Emphysema Treatment
Bronchoscopic lung volume reduction (BLVR) with endobronchial valves is a novel therapy for severe emphysema. This is a minimally invasive solution for lung volume reduction in patients who are already on optimal medical management.
In the right patients, endobronchial valve treatment can significantly improve the symptoms of emphysema by redirecting air away from diseased parts of the lung to healthier parts, reducing hyperinflation and improving lung function.1
Why Choose the Spiration Valve System?
A Decade of Clinical Experience
The Spiration® Valve System has been available in the US Market, since 2008, under a Humanitarian Device Exemption (HDE) FDA approval, for the management of post-surgical prolonged air leaks. Physicians continue to choose the Spiration Valve System for emphysema treatment not only because of their trust and familiarity with the product after years of clinical practice but also because it has been proven in multiple clinical trials that it is a safe and effective treatment option for patients with severe emphysema.
The EMPROVE Clinical Trial demonstrated the Spiration Valve System is a safe and effective treatment option for patients with severe emphysema and little-to-no collateral ventilation.1

Low 14.2% rate of serious pneumothorax
Short 24 min. procedure time which may reduce the risk of serious adverse events1,5
52.8% reduction of the target lobe volume


6.3% reduction in hyperinflation
As measured by RV/TLC ratio
12.1% significant increase in FEV1


29.6% decrease in dyspnea
as determined by mMRC score
9.5 point reduction in SGRQ* score
Demonstrating a substantial and clinically-meaningful improvement

Patient Stories
Real Results from Real Patients

“[This procedure] is marvelous. It changes your life…it is a second chance at life.”
Watch Lucie’s Story
“A few days afterwards, I started feeling normal again, like a mother, a grandmother, and just getting back to doing things that I love to do.”
Watch Marion’s Story
“I can walk up the stairs, and I’m not out of breath so frequently. I had a major improvement on my life.”
Watch Ronny’s Story
“The term ‘life-changing’ comes to mind. At least for me, I’ve gotten a lot back that I had lost.”
Watch Chris and Jodi’s StoryPotential complications which may be associated with bronchoscopy and/or the Spiration Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, dyspnea and in rare cases, death. Prior to using the Spiration Valve System, please review the full list of prescriptive information at https://svs.olympusamerica.com/prescriptive-information for additional information on indications, contraindications, warnings, precautions and potential complications.
Procedure Overview
Initial Patient Screening
Proper patient management is of the utmost importance for endobronchial valve treatment. Due to the complexity of this procedure, each step of the patient management process, from the initial patient screening to the post-procedure
re-evaluation, requires a thorough assessment to ensure successful outcomes.
Pre-procedure
Medical History &
Physical Exam
- Patient is 18 years of age or older
- Patient is not an active smoker
- Patient is diagnosed with GOLD stage III or IV COPD (severe emphysema)
- Patient does not have a known or suspected sensitivity or allergy to nickel
- Patient meets the criteria of the ATS/ERS Guidelines for Management of Stable COPD
- Patient is on optimized medical management within standard of care
Pulmonary Function Testing
& Medical Work Up
- FEV1: >15 and ≤ 45% predicted
- Residual Volume (RV): >150% predicted
- Total Lung Capacity: ≥100% predicted
- 6MWD: >100 meters
- DLCO: > 20%
- mMRC: ≥ 2
Radiographic
Assessment
- Fissure Integrity of ≥ 90% completeness of the fissure separating the target lobe
- High Heterogeneity of ≥ 10-point severity difference between target and the ipsilateral lobe
- Emphysema Severity – target lobe with ≥ 40% emphysema involvement
If the patient meets the recommended selection criteria for best results, they can be considered a candidate for Spiration Valve System treatment.
If the patient does not meet the recommended selection criteria, other options for treatment should be discussed and agreed upon.
Download Selection Criteria
Peri-procedure
Medication
- Prophylactic antibiotics and steroids (at physician’s discretion)
- Provide nebulized bronchodilators 20 minutes prior to case
- Provide post-procedural bronchodilators immediately after procedure in recovery area and then every 4–6 hours as needed
Anesthesia
- General anesthesia (GA) and sedation are both acceptable for this procedure
- Determination of GA vs. sedation depends on local preference and experience and pre-anesthesia patient evaluation
Airway Management
- For reliable oxygenation and ventilation, an endotracheal tube that is a minimum size 8 mm or 8.5–9 mm is preferred

The Spiration® Valve System instructional videos provide information about the safe and effective use of the product.
Watch Instructional Videos
Post-procedure
Pneumothorax Management
- Monitoring for pneumothorax with a chest x-ray and/or chest CT scan is crucial since the majority of pneumothoraces occurs within 72 hours after the procedure
- A dedicated pneumothorax cart/tray should always be in the patient room after the procedure
Patient Expectations
- It is important that patients know the Spiration® Valve System procedure is not a cure for their emphysema but can provide relief of their symptoms
- While some patients can wake up feeling an almost immediate sense of improvement, others can take up to as long as 3 months to feel the full benefit from the valves
Follow Up
- The patient follow up plan is determined by the treating physicians and dependent upon the results of the procedure
Frequently Asked Questions
- Non-serious (Moderate) PTX led to Respiratory failure
- Serious PTX. Pneumonia occurred 7 days later
- Non-serious (Severe) PTX associated with pneumonia
- Non-serious (Moderate) associated with respiratory failure
- Serious PTX associated with respiratory failure
- Non-serious (Moderate) led to pneumonia 7 days later
- Serious PTX associated with pneumonia
| Delta Baseline – 3 Months | FEV1 (ml) | mMRC (points) | CAT (points) | SGRQ (points) |
|---|---|---|---|---|
| Serious PTX (N=14) | 129 ±222 | -0.62 ±1.26 | -4.3 ±7.5 | -10.3 ±15.2 |
| No PTX (N=99) | 124 ±164 | -0.49 ±0.96 | -3.0 ±7.0 | -8.2 ±17.8 |
| P- value between groups | 0.643 | 0.921 | 0.893 | 0.802 |
| UPPER LOBES | 9mm valves only | 9 + mix of valves | No 9mm valves |
|---|---|---|---|
| Serious PTX | 4/22 | 9/35 | 6/22 |
| Non-serious PTX | 3/22 | 2/35 | 1/22 |
| LOWER LOBES | 9mm valves only | 9 + mix of valves | No 9mm valve |
| Serious PTX | 1/10 | 0/20 | 0/4 |
| Non-serious PTX | 1/10 | 6/20 | 0/4 |
| ALL LOBES | 9mm valves only | 9 + mix of valves | No 9mm valves |
| Serious PTX | 5/32 | 9/55 | 6/26 |
| Non-serious PTX | 4/32 | 8/55 | 1/26 |
| Measure | MCID | EMPROVE | LIBERATE |
|---|---|---|---|
| SGRQ | 4.0 points | 50.5% | 56.2% |
| mMRC | 1.0 point | 48.9% | 47.8% |
| Delta Baseline – 6 Months | FEV1 (ml) | TLV (L) | RV (L) | mMRC (points) | SGRQ (points) |
|---|---|---|---|---|---|
| Fissure Integrity ≥95% (N=87) | 112 ±159 | -1.02 ±0.75 | -0.49 ±0.85 | -0.66 ±1.04 | -9.5 ±16.7 |
| Fissure Integrity 90-94% (N=19) | 38 ±115 | -0.74 ±0.60 | -0.002 ±0.75 | -0.58 ±1.02 | -1.9 ±18.0 |
| P- value between groups | 0.0576 | 0.1607 | 0.0224 | 0.7601 | 0.0853 |
PulmonX makes this claim based on a retrospective analysis of data collected by the Thoraxklinik in Heidelberg, Germany.2 The dataset for this analysis included both SVS and PulmonX Zephyr valves, so this is not strictly a PulmonX-only claim. As such, it is a bit disingenuous to claim a benefit of improved mortality based on a single, retrospective study, let alone a benefit tied to a specific valve, but the data is an encouraging signal that BLVR may be associated with improved mortality, independent of valve used.
| PO2 | Treatment | Control |
|---|---|---|
| Baseline | 67.89 | 67.95 |
| Delta at 3 months | -2.34* | 0.38 |
| Delta at 6 months | -1.80* | 0.72 |
| PCO2 | Treatment | Control |
|---|---|---|
| Baseline | 40.16 | 40.94 |
| Delta at 3 months | -0.81 | -0.60 |
| Delta at 6 months | -0.59 | 0.75 |
The markedly lower pneumothorax rate in our study may be attributable to a very conservative post-operative care regimen, with patients staying in-hospital for a median of 6 days post-intervention and exposed to limited activities of daily living, which has been previously shown to reduce pneumothorax incidence.
In this study, the average emphysema severity in the ipsilateral lobe was 34.6%, with an emphysema heterogeneity between lobes of 28.4% (see Table 2). In contrast, the ipsilateral lobe emphysema severity in the TRANSFORM and LIBERATE studies was 45.4% and 47.5%, respectively, and this may account for the comparatively lower pneumothorax rate seen in the REACH study. Gompelmann, et al2., have also noted that with greater emphysematous destruction of the untreated ipsilateral lobe there is an increased incidence of pneumothorax.
Dr. Criner at Temple recently presented his order of importance when selecting a target lobe. That list, in order of priority is:
- Fissure integrity (Collateral ventilation)
- Emphysema destruction
- Perfusion
- Volume
- Degree of heterogeneity
- Anatomy
- REACTIVE ONLY: During the EMPROVE study an overnight stay was required. If the investigator had a high suspicion of potential pneumothorax, they were allowed to keep the patient longer, per their medical discretion. The mean hospital stay in EMPROVE was 3.81 days, with a median of one day.
Olympus has designated services and programs available to assist you with all of your reimbursement quefstions and needs related to the Spiration Valve System.
Contact Information:
Hours: 9:00 am – 5:00 pm Eastern Standard Time
Phone: 855-428-7346
Email: SpirationValveReim@olympus.com
Need Basic Reimbursement Information?
Feel free to call or email us and our trained staff can assist you with questions on billing, coding, and reimbursement for the Spiration Valve System.
Experienced coding experts are available to help providers navigate your case-specific denials and prior authorizations. Our experts can assist with communication and paperwork between your payers, and then update you on case-specific decisions through a secured provider portal.
If you are interested, please contact the Spiration Reimbursement Helpline to learn more about this service.
NOTE: A signed Business Associates Agreement is required for this level of service.
2. Gompelmann D, et al. Respiration. 2019;97(2):145-152. doi: 10.1159/000492274.
3. Li S, et al. Respiration. 2018:1-12. doi:10.1159/000494327
Are You Looking for More Information?
Fill out the form to submit a question to Olympus regarding the Spiration Valve System.
Submit a QuestionDownload Resources
Instructional Videos
Prescriptive Information
Management of Prolonged Airleaks
Referring
Physicians
Patient
Information
- Criner GJ et al., 2019 Improving Lung Function in Severe Heterogenous Emphysema with the Spiration® Valve System (EMPROVE): A mliticenter, Open-Label, Randomized, Controlled Trial. Am J Respir Crit Care Med, 2019. https://doi.org/10.1164/rccm.201902-0383OC
- Centers for Disease Control. Chronic Obstructive Pulmonary Disease: Basics About COPD. https://www.cdc.gov/dotw/copd/index.html. Accessed December 3, 2018.
- https://www.nice.org.uk/guidance/ipg600/resources/endobronchial-valve-insertion-to-reduce-lung-volume-inemphysema-pdf-1899873854992069. Accessed 2018.
- Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial Valves for Endoscopic Lung Volume Reduction: Best Practice Recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration. 2017;93(2):138-150.
- Schuhmann M, Raffy P, Yi Y, et al. CT Predictors of Response to Endobronchial Valve Lung Reduction Treatment: Comparison with Chartis. Am J Respir Crit Care Med 2015; 191(7):767-774; doi:10.1164/rccm.201407-1205OC.
- Hogarth DK, Delage A, Zgoda MA, Reed MF. American Thoracic Society International Conference Abstracts. American Thoracic Society; 2018:A7754-A7754. doi:10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A7754.
- Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol. 2014;21(4):288-297. doi:10.1097/LBR.0000000000000110.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD, 2019. http://golcopd.org
- Michael Reed, MD and Jennifer Toth, MD, the authoring physicians of this presentation, are paid consultants to Olympus Corporation of the Americas.