Olympus Announces FDA Clearance of RenaFlex™, Its First Single-use Flexible Ureteroscope
The RenaFlex™ Single-use Ureteroscopy System can be used to perform diagnostic and therapeutic procedures within the urinary tract

CENTER VALLEY, Pa., (April 2, 2024) – Olympus, a global medical technology company committed to making people’s lives healthier, safer and more fulfilling, announced today U.S. FDA 510(k) clearance of its first single-use ureteroscope system, RenaFlex™, with full market availability to be announced at a later date.
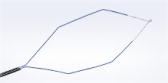
The RenaFlex single-use flexible ureteroscope system is used to access and visualize the urinary tract to diagnose and treat urinary diseases and disorders, such as kidney stones. The RenaFlex system is intended to visualize organs, cavities, and canals in the urinary tract (urethra, bladder, ureter, calyces and renal papillae) via transurethral or percutaneous access routes. The RenaFlex ureteroscope can be used with endotherapeutic accessories to perform diagnostic and therapeutic procedures within the urinary tract.
Regardless of stone size, composition or location, Olympus offers a broad range of stone management solutions to confidently approach every patient or procedure, at any site of care. With the addition of the RenaFlex system, the stone management solution portfolio offers physicians options to be ready for every stone. Key benefits of the RenaFlex Single-use Ureteroscope System include:
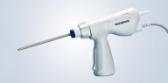
- Maneuverability:
- Lightweight, ergonomic ureteroscope handlei
- Flexible shaft with stiff and passive bending sectionsii
- Visualization
- High-quality, consistent visualization during each procedureiii
- Advanced camera chip within the distal tip, 120-degree field of view with auto white balancing and brightnessiv
- Therapeutic Active articulation: 270-degree up/down angulationv
- 9.5Fr outer diameter with 3.6Fr working channelv
- Simple set-up that requires minimal O.R. space.
“Olympus is your trusted stone management partner and now is positioned to provide access to a broad range of high-quality ureteroscopes, including reusable and single-use flexible scopes to rigid and semi-rigid scopes, powerful lithotripsy solutions and simplified stone removal devices,” said Glen Branconier, Vice President, Business Unit Leader for Urology/Gynecology at Olympus Corp. of the Americas. “We are excited and privileged to provide urologists and Health Care Providers with the tools they need to confidently care for their patients at any site of care.”
As with all medical devices please refer to your healthcare provider and the RenaFlex™ Single-use Ureteroscope system Instructions for Use.vi,vii
The RenaFlex Single-use Ureteroscope works in combination with the compatible CV-S1 Video System Center for flexible Single-use Endoscopes. For safe and proper use follow the manufacturer’s instructions for use handling and operating of the RenaFlex Single-use Ureteroscope and the CV-S1 Video System Center. If used or handled in an improper manner, there is a risk of patient and/or operator injuries, burns, infections, bleeding, and/or perforation, and/or equipment damage.
Visit the urology product page for more information about the complete line of Olympus urology solutions.
# # #
About Olympus
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states.
For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world.
Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America. For more information, visit olympusamerica.com.
i Data on File DN0054301
ii Data on File M160-063R-00
iii Data on File DN0057620
iv Data on File DN0057516
v Data on File M160-063R-00
vi IFU PN0022005_AA
vii IFU PN0017751_AB