iTind System for BPH Treatment Reaches Two Major Milestones: First Commercial Treatments and CMS Reimbursement Code Assigned
First iTind Procedures to Relieve Symptoms of Enlarged Prostate, without Compromise to Sexual Function, Are Performed; iTind Procedure Also Receives Medicare Outpatient Billing Codes for Reimbursement
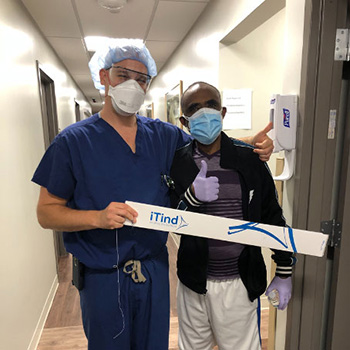
Dr. Jonathan Warner (left) with Dr. Seyoum Gebremariam, one of the first patients treated for symptoms of benign prostatic hyperplasia, also known as enlarged prostate, using the minimally invasive iTind device.
CENTER VALLEY, Pa., (November 17, 2020) – Olympus announced today the first commercial treatment of Benign Prostatic Hyperplasia (BPH), known as an enlarged prostate, using the novel iTind System. Jonathan Warner, MD, Urologic Surgeon and Assistant Professor in the Division of Urology and Urologic Oncology for the Department of Surgery at Emanate Health Foothill Presbyterian Hospital in Glendora, CA and Kenneth Kernen, MD, Chief of Urology and Associate Professor of Surgery at Beaumont Health in Michigan, were the first two urologists to treat patients using this new minimally invasive technique.
“I’m excited to have been among the first doctors in the U.S. to place an iTind device and gratified to bring relief to a patient who had been suffering from the symptoms of an enlarged prostate,” said Dr. Jonathan Warner. “The iTind is a great new option for men looking for a less risky way to manage their BPH symptoms. It’s straightforward and comes with no side effects, so it’s a win-win for the doctor and the patient.”
Dr. Warner’s first patient, Seyoum Gebremariam, Ph.D., a Los Angeles-based researcher, said the treatment “has made a clear difference” in resolving symptoms he had suffered for 10 years, including a persistent urge to urinate. Dr. Gebremariam carefully researched his options for treatment, reading all the information he could find online, and he opted for the iTind procedure based on the low risks involved. “The risk is very minimal, and the advantage is great,” said Dr. Gebremariam. “I can void my bladder and am no longer bothered by the constant feeling that I need to use the toilet, which is very disruptive to your life. I’m happy with my choice and think this treatment is remarkable.”
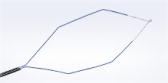
Treatment with the iTind System is straightforward and avoids complications associated with prescription medications, surgery, or permanent implants. Patients are able to return home following the brief placement procedure. The iTind device has three flexible nitinol (nickel titanium alloy) struts that gently expand to create channels that reshape the prostate, allowing urine to flow. After one week, the device is removed, and patients experience near-instant relief. Placement and retrieval can be done in a medical office setting. Unlike other treatments for BPH, iTind has been shown to not compromise sexual function.
To help support more patients being treated with the iTind System, the Centers for Medicare & Medicaid Services (CMS) published a final rule that establishes a new technology “C Code” that will facilitate payment when the iTind procedure is performed in an outpatient hospital department or ambulatory surgical center (ASC). New technology APC (ambulatory payment classification) codes provide payment guidance for new procedures that cannot be appropriately reported using existing codes. iTind was FDA authorized under the de Novo approval process for new technologies on February 25, 2020.
BPH is one of the most common diseases in aging men and the most common cause of lower urinary tract symptoms (LUTS).i According to the American Urological Association, BPH is a condition that 8 out of 10 men will face in their lifetimes.ii In a survey of the general population that Olympus conducted for Prostate Health Awareness month in September 2020, Olympus found that erectile dysfunction tops the list of patient concerns about treating BPH. Seventy-five percent of respondents said that they would be likely or very likely to request a treatment for BPH that would relieve the symptoms of the disorder without compromising sexual function.
The iTind was developed and is manufactured by Israeli-based Medi-Tate and distributed worldwide by Olympus. More information for prospective patients and physicians interested in the iTind System can be found at www.iTind.com.
For years Olympus has been an innovator in developing surgical treatment options for BPH, with electrodes for resection, vaporization and enucleation of the prostate. Having reached another major milestone with the iTind System, Olympus will continue to be a global market leader in men’s health.
# # #
About Olympus
Olympus is passionate about the solutions it creates for the medical, life sciences, and industrial equipment industries.
Olympus’ Medical business uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus’ Medical portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of endotherapy instruments. For more information, visit www.olympus-global.com.
About Medi-Tate
Medi-Tate is an Israeli medical device company that deals in the R&D, manufacture and sale of innovative solutions for the treatment of Lower Urinary Tract Symptoms (LUTS) with a mission to commercialize a safe and effective, office-based solution for BPH.
i Lim KB. Epidemiology of clinical benign prostatic hyperplasia. Asian J Urol. 2017;4(3), 148–151. doi:10.1016/j.ajur.2017.06.004
2 Medical Student Curriculum: Benign Prostatic Hyperplasia (BPH). Auanet.org. https://www.auanet.org/education/auauniversity/medical-student-education/medical-student-curriculum/bph. Published May 2013, Updated July 2016. Accessed March 3, 2020.