Olympus Announces Release of Redesigned Guide Sheath Kit
The latest generation of guide sheath provides greater flexibility and offers physicians better access to the peripheries of the lungs

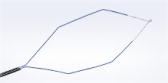
Olympus announces the release of its latest generation of Guide Sheath Kit that is designed to facilitate access to peripheral pulmonary lesions.
CENTER VALLEY, Pa. (April 13, 2022) — Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, announced the release of its latest generation of Guide Sheath™ Kit, designed to facilitate access to lesions in peripheral regions of the lung via bronchoscopy with radial EBUS (endobronchial ultrasound).
The Guide Sheath Kit 2 is used as part of Olympus' robust lung cancer portfolio that facilitates a physician's ability to precisely locate and sample lesions. The development of targeted therapies for lung cancer makes collecting adequate tissue samples a priority to assure all molecular marker tests can be completed to provide personalized, targeted treatment.
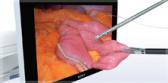
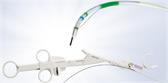
Called the Guide Sheath Kit 2, the new version of the device features a braided wire inside the guide sheath, providing kink resistance and greater flexibility1. The single-use kit includes a 1.5 mm biopsy forceps and 1.4 mm cytology brush for specimen collection and clip-type stoppers designed to simplify the setup and positioning of an ultrasonic probe and endotherapy devices before and during a procedure.
Other benefits of the Guide Sheath Kit 2 in support of high-precision pulmonary examinations include:
- The guide sheath can reach bronchi branches beyond the distal end of the bronchoscope, allowing physicians to advance endoscopic devices further into the periphery to access pulmonary lesions.1
- An improved contrast to the tip of the guide sheath allows for easier positioning under fluoroscopic visualization of the distal end of the sheath.1
- Once the instrument is positioned, physicians can repeatedly take samples while leaving the guide sheath in place, allowing for a more efficient examination.1
"As a leader in the field of pulmonology solutions, Olympus is excited to announce that it has strengthened its peripheral bronchoscopy offerings with the redesigned Guide Sheath Kit 2," said Swarna Alcorn, Vice President for Respiratory Business Unit at Olympus America Inc. "Early diagnosis and staging of lung cancer is crucial and devices like the Guide Sheath Kit 2 give physicians access to the periphery of the lung where cancer is found in its earliest stages."2
When lung cancer is diagnosed at a localized stage, the 5-year survival rate is about 60%, but only 24% of lung cancers are diagnosed at an early enough stage, according to the American Cancer Society.3 The United States Preventive Services Task Force recommends that adults ages 50 to 80 who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years receive annual low-dose computed tomography (LDCT) scans4. A person's pack-year is determined by the number of packs of cigarettes smoked per day multiplied by the number of years they smoked. For instance, 20 pack-years is equal to one pack a day for 20 years or two packs a day for 10 years.
Guide Sheath Kit 2 is part of Olympus' larger lung cancer solutions for diagnosis and staging to help guide patient care. The portfolio includes solutions such as the SPiN Thoracic Navigation System™ and Radial EBUS to aid in accessing and sampling peripheral pulmonary lesions as well as Endobronchial Ultrasound-guided Transbronchial Needle Aspiration (EBUS-TBNA) that uses ultrasound to see beyond the walls of the airways to detect the precise location of nodules and lymph nodes.
Complications from bronchoscopy are rare and most often minor, but if they occur, they may include breathing difficulty, vocal cord spasm, hoarseness, slight fever, vomiting, dizziness, bronchial spasm, infection, low blood oxygen, bleeding from biopsied site, or an allergic reaction to medications. Only rarely do patients experience other more serious complications (for example, collapsed lung, respiratory failure, heart attack and/or cardiac arrhythmia).
Visit the Olympus website for more information about the Guide Sheath Kit 2 or visit the pulmonary product page for more information about Olympus's complete product line.
###
About Olympus
Olympus is passionate about creating customer-driven solutions for the medical, life sciences, and industrial equipment industries. For more than 100 years, Olympus has focused on making people's lives healthier, safer and more fulfilling by helping to detect, prevent, and treat disease; furthering scientific research; and ensuring public safety. In its Endoscopic Solutions business, Olympus uses innovative capabilities in medical technology, therapeutic intervention and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance the quality of life for patients and their safety. Starting with the world's first gastrocamera in 1950, Olympus' Endoscopic Solutions portfolio has grown to include endoscopes, laparoscopes, and video imaging systems, as well as system integration solutions and medical services. For more information, visit https://medical.olympusamerica.com.
- Data on file with Olympus (March 6, 2020)
- American Cancer Society, "Cancer A-Z: About Lung Cancer" Accessed March 31, 2022
- American Cancer Society, "Cancer Facts and Figures 2022" Accessed March 11, 2022
- U.S. Preventive Services Task Force "Final Recommendation Statement Lung Cancer: Screening" Published March 9, 2021. Accessed March 11, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening