Olympus Strikes Exclusive Deal to Distribute Ultravision Surgical Smoke Management System in the U.S.
Ultravision™ Controls Aerosolized Particles in Laparoscopic Surgery, Leading to Cleaner Air in the Operating Room
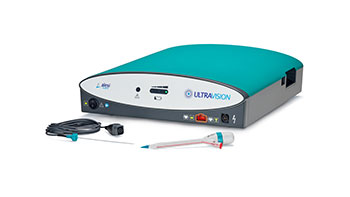
Olympus announces that it has entered into an exclusive agreement with Alesi Surgical Limited to distribute the 510(k)-cleared Ultravision surgical smoke control system in the U.S.
CENTER VALLEY, Pa., (September 10, 2020) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today that it has entered into an exclusive agreement with Alesi Surgical Limited to distribute the 510(k)-cleared Ultravision surgical smoke control system in the U.S.
Surgical smoke is a gaseous byproduct of tissue treated with electrical surgical devices, which are used for cautery, cutting, and ablation, among other uses. If not properly managed, surgical smoke can be hazardous to the health of people working within the surgical suite. Physicians and nurses recommend using a smoke management device to reduce the risk of potential exposure to aerosolized contaminants,i,ii and two U.S. states have passed laws mandating that hospitals install local exhaust ventilation systems in procedure rooms to control smoke and reduce the risk of staff exposure, with other state legislation pending.
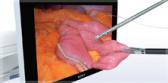
In the United States, Ultravision is cleared for use in laparoscopic and open surgery. Ultravision suppresses the aerosolization of surgical smoke and mist using the highly characterized process of electrostatic precipitation. Clinical research has shown that Ultravision improves visibility, prevents the release of surgical smoke into the operating room, reduces patient CO2 exposure and facilitates “low pressure” laparoscopic surgery.iii,iv
“Using an effective surgical smoke management device is essential to minimizing the risk of exposure for surgical teams in the operating room,” said Ross “Rusty” Segan, MD, MBA, FACS, Chief Medical Officer for Olympus Corporation. “Healthcare professionals are rightly concerned about risks posed by bioaerosols, especially during the pandemic. However, we must find safe ways to continue performing surgical procedures using minimally invasive techniques because of the significant clinical benefits this type of surgery offers patients. With its unique and highly characterized mode of action, Ultravision offers an innovative means to control bioaerosols during laparoscopic surgery.”
Ultravision addresses three major challenges in managing surgical smoke:
- Managing Surgical Smoke: In laparoscopic surgery, unlike other smoke control devices, Ultravision has been independently shown to remove particulates as small as 0.007µm - smaller than any known virus – from the atmosphere, without the need for CO2 exchange.v The system is designed to prevent the need to vent surgical smoke into the perioperative environment.
- Improving Workflow: By rapidly accelerating the natural sedimentation of surgical smoke, Ultravision provides a continuously clear visual field during laparoscopic surgery. Less smoke in the visual field reduces the need to pause procedures while waiting for smoke to clear, removing and replacing CO2 gas, or cleaning the camera lens. This helps the surgical team operate accurately and efficiently.
- Minimizing CO2 Exposure to the Patient: Because it is designed to clear the visual field without the need to release smoke from within the pneumoperitoneum (abdominal cavity), Ultravision minimizes the amount of cold, dry CO2 that a patient is exposed to during the procedure. Excessive CO2 exposure is associated with undesirable local and systemic clinical effects. Furthermore, by providing a constant, stable pneumoperitoneum, Ultravision facilitates low pressure surgery, which has been demonstrated to enhance patient recovery after general surgery and gynecological procedures.vi,vii
“Ultravision is the only technology that rapidly and continuously clears the smoke from the visual field without requiring CO2 exchange and filtration. It is the only product that provides maximum view with minimum carbon dioxide exposure,” said Dominic Griffiths, PhD, CEO of Alesi Surgical. “Given the immense resources and expertise of Olympus, this exciting partnership will greatly accelerate the ability of U.S. hospitals to access Ultravision and provide its benefits to staff and patients.”
“Ultravision is a great complement to the Olympus portfolio of surgical energy products, providing a proven solution for controlling surgical smoke and supporting staff safely in the OR,” said Phil Roy, Vice President and General Manager, Global Surgical Devices at Olympus Corporation of the Americas. “We are very excited about this new distribution agreement with Alesi, which is an important step in expanding our comprehensive offering for existing and future customers.”
# # #
About Olympus
Olympus is passionate about the solutions it creates for the medical, life sciences, and industrial equipment industries, as well as cameras and audio products.
Olympus’ Medical business uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus’ Medical portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of endotherapy instruments. For more information, visit www.olympus-global.com.
About Alesi Surgical
Alesi Surgical develops and commercializes products that improve the safety, efficiency and outcomes of advanced surgical procedures. The company was founded in 2009 as a spin-out from the Welsh Institute for Minimal Access Therapy (WIMAT), with the vision of becoming a world leader in surgical devices. Part of Cardiff University, WIMAT runs multi-disciplinary training courses across a range of surgical and medical specialties and is the busiest multi-disciplinary training center in the United Kingdom. Alesi is a privately owned company, backed by leading European healthcare investors IP Group PLC, Development Bank of Wales, Panakes Partners SPA and Earlybird Health Technologies GmbH.
For more information, visit www.alesi-surgical.com or call +44(0) 29 202 910 22.
i da Costa, KM, Saxena, AK. Coronavirus disease 2019 pandemic and identifying insufflators with desufflation mode and surgical smoke evacuators for safe CO2 removal. Asian J Endosc Surg. 2020; 1– 5. https://doi.org/10.1111/ases.12834
ii Chavis, S, et al. Clearing the Air About Surgical Smoke: An Education Program. AORN Journal. 103(3):289-296 https://doi.org/abs/10.1016/j.aorn.2016.01.007
iii Ansell J, et al. Electrostatic precipitation is a novel way of maintaining visual field clarity during laparoscopic surgery: a prospective double-blind randomized controlled pilot study. Surg Endosc. 2014;28(7):2057-2065. doi:10.1007/s00464-014-3427-8;
iv Levine D, et al. Electrostatic Precipitation in Low Pressure Laparoscopic Hysterectomy and Myomectomy. Manuscript submitted for publication.
v Alesi Surgical verification report DREP-002, performed by Cardiff University.
vi Hua, J et al. Low-pressure versus standard-pressure pneumoperitoneum for laparoscopic cholecystectomy: a systematic review and meta-analysis. Am J Surg. 2014 Jul;208(1):143-50. doi: 10.1016/j.amjsurg.2013.09.027.
vii Radosa, J et al. Impact of different intraoperative CO2 pressure levels (8 and 15 mmHg) during laparoscopic hysterectomy performed due to benign uterine pathologies on postoperative pain and arterial pCO2: a prospective randomised controlled clinical trial. BJOG. 2019 Sep;126(10):1276-1285. doi: 10.1111/1471-0528.15826.