Olympus Launches Spiration® Valve System for the Endobronchial Treatment of Severe Emphysema
A novel, minimally invasive therapy blocks airflow to the diseased part of the lung and is shown to improve lung function and quality of life

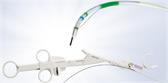
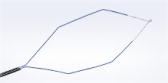
The FDA-approved Olympus Spiration® Valve System is now market available for the therapeutic treatment of emphysema. Placed in targeted airways of the lung during a short bronchoscopic procedure, the Spiration Valve is an umbrella shaped device that improves breathing by redirecting air from diseased parts of the lungs to healthier parts, enabling healthier tissue to expand.
CENTER VALLEY, Pa., (January 22, 2019) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today the market availability of its FDA-approved Spiration Valve System (SVS) for the treatment of severe emphysema, a progressive form of Chronic Obstructive Pulmonary Disease (COPD). Prominent guidelines1,2, now recommend minimally invasive bronchoscopic lung volume reduction using endobronchial valves as an alternative treatment option for severe emphysema to more invasive options, such as surgery.
Placed in targeted airways of the lung during a short bronchoscopic procedure, the Spiration Valve is an umbrella shaped device that improves breathing by redirecting air from diseased parts of the lungs to healthier parts, enabling healthier tissue to expand. SVS therapy may lead to volume reduction in the treated part of the lung, allowing the healthier tissue in the remaining portion of the lung to function more effectively.3 With improved breathing, patients suffer less from breathlessness and fatigue and, as a result, can do more in their daily lives and enjoy an improved quality of life.
FDA approval of the SVS was based on results of the EMPROVE clinical trial demonstrating that patients treated with the SVS benefited from statistically significant and clinically meaningful improvements in lung function and quality of life compared to standard of care medical management. The results showed that the SVS offers a favorable risk benefit profile, with a short procedure time4. Previous clinical study results5 have shown that shorter procedure times may reduce the risk of adverse events. Serious adverse events observed in the study include COPD exacerbations, pneumothorax, pneumonia and death.
“The EMPROVE trial corroborates and substantiates other data that shows how important it is to recognize that hyperinflation has a negative outcome on patients physiologic function, their quality of life, and even their survival,” said Dr. Gerard Criner, MD, Professor and Founding Chair of the Department of Thoracic Medicine and Surgery at the Lewis Katz School of Medicine, Temple University. “By having a potent therapy that significantly reduces lung hyperinflation, and is durable over time, you can start to see patients making truly meaningful improvements and achieving clinical outcomes such as improvements in lung function and quality of life.”
“The SVS offers significant benefit to a certain subpopulation of patients with emphysema,” said Dr. Kirk Voelker, MD, Interventional Pulmonologist and Director of Clinical Research at Sarasota Memorial Hospital. “There were a low number of complications, such as pneumothorax and pneumonia, which are all easily treated, but, importantly, we saw the durable effects of the therapy lasting through the follow-up year with significant improvement in quality of life.”
The SVS is approved for use in the treatment of emphysema in the U.S., E.U. countries, Australia and New Zealand.
According to the Centers for Disease Control and Prevention, COPD affects more than 15 million people, including the 3.5 million who have emphysema, and is the fourth leading cause of death in the U.S.6 The SVS is an important new therapy for adult patients with shortness of breath and hyperinflation associated with severe emphysema in regions of the lung that have evidence of low collateral ventilation.
“We are very excited to add the Spiration Valve System to our broad portfolio of respiratory devices and bronchoscopes,” said Kurt Heine, Group Vice President of the Endoscopy Division at Olympus America Inc. “The SVS provides physicians a minimally invasive option for treating severe emphysema, allowing patients to find relief from debilitating symptoms.”
For more information about the SVS, please visit us at http://svs.olympusamerica.com.
# # #
About Olympus Medical Systems Group
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our nearly 100-year history, Olympus has focused on being true to society and making people's lives healthier, safer and more fulfilling.
Our Medical Business works with health care professionals to combine our innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing with their skills to deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance quality of life for patients. For more information, visit medical.olympusamerica.com.
1 2019 Global Strategy for the Diagnosis, Management, and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2018. http://goldcopd.org
2 National Institute for Health Care and Excellence. 2017. Endobronchial valve insertion to reduce lung volume in emphysema; Interventional procedures guidance [IPG600]. https://www.nice.org.uk/guidance/ipg600/resources/endobronchial-valve-insertion-to-reduce-lung-volume-in-emphysema-pdf-1899873854992069
3 Spiration Valve System. 2018. Summary of Safety and Effectiveness.
4 Criner GJ, Delage A, Voelker KG, for the EMPROVE Trial Investigator Group. The EMPROVE Trial - a Randomized, Controlled Multicenter Clinical Study to Evaluate the Safety and Effectiveness of the Spiration® Valve System for Single Lobe Treatment of Severe Emphysema. American Thoracic Society International Conference Abstracts. 2018:A7753-A7753. doi:10.1164/ajrccm-conference.2018.197.1_ MeetingAbstracts.A7753.
5 Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol. 2014;21(4):288-297.
6 Chronic Obstructive Pulmonary Disease: Basics About COPD, Centers for Disease Control. https://www.cdc.gov/dotw/copd/index.html, June 5, 2018