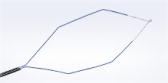
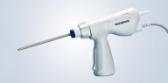
Olympus Launches New Gynecologic Power Morcellator for Its Contained Tissue Extraction System
Minimally invasive surgery option for low-risk hysterectomy, myomectomy patients
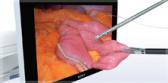
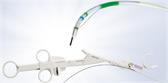
CENTER VALLEY, Pa., (November 30, 2022) - Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, announced today the market launch of the moresolution™ Power Morcellator, which is manufactured by TROKAMED GmbH and is available in the U.S. through a distribution agreement with Olympus America, Inc. The moresolution Morcellator is designed for advanced gynecologic procedures with large, calcified tissue specimens.
Current FDA Guidance on power morcellator labeling requires that all power morcellators marketed in the U.S. be used with a compatible, FDA-cleared containment device.i The Olympus Contained Tissue Extraction System comprises the PneumoLiner™, a first-of-its-kind containment device, and the moresolution Morcellator, which together provide select patients a laparoscopic surgical option as an alternative to open hysterectomy or open myomectomy for tissue removal.
Contained tissue extraction is not indicated for use with women who have a known or suspected malignancy. The FDA advises that power morcellation should not be used for removal of uterine tissue containing suspected fibroids in patients who are post-menopausal or over 50 years of age or who are candidates for en bloc tissue removal vaginally or via a mini-laparotomy incision.
Features and benefits of the moresolution Morcellator include:
- Ability to cut dense or calcified fibroids
- Efficient cutting may help save time in the operating room
- Lightweight handpiece designed to reduce surgeon fatigue
- Safety features include the option to keep the device in 'Cut' or 'No Cut' mode
- Autoclaveable and reusable components may support efforts for cost reduction
"We are excited to offer a new power morcellation solution for minimally invasive gynecological procedures," said Richard Reynolds, President of the Medical System Group at Olympus America, Inc. "Olympus supports the safe use of power morcellators with a contained extraction system according to FDA guidance. This device reflects a response to our customers' requests for more power and control during the procedure. This particular device works very well on very dense, calcified tissue, which is difficult to cut with other devices."
Gynecological societies stand behind the FDA guidance on the safe use of power morcellation. The American Congress of Obstetricians and Gynecologists (ACOG) points out that hysterectomy or myomectomy performed by open surgery is associated with increased morbidity when compared with minimally invasive approaches such as laparoscopy.ii The American Association of Gynecologic Laparoscopists (AAGL) supports continued use of morcellation given appropriate patient selection and surgical technique to minimize established risks and optimize benefits of the procedure based on current evidence.iii
# # #
About Olympus
As a leading medical technology company, Olympus delivers innovative medical technology, therapeutic intervention, and precision manufacturing used in diagnostic, therapeutic, and minimally invasive procedures. For more information, visit https://medical.olympusamerica.com.
- UPDATE: Perform Only Contained Morcellation When Laparoscopic Power Morcellation Is Appropriate: FDA Safety Communication. FDA.gov. Published December 29, 2020. Accessed November 15, 2022. https://www.fda.gov/medical-devices/safety-communications/update-perform-only-contained-morcellation-when-laparoscopic-power-morcellation-appropriate-fda
- American College of Obstetricians and Gynecologists' Committee on Gynecologic Practice. Uterine Morcellation for Presumed Leiomyomas. Number 822. Published March 2021. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2021/03/uterine-morcellation-for-presumed-leiomyomas. Accessed November 19, 2022
- The Tissue Extraction Task Force Members. Morcellation during Uterine Tissue Extraction: An Update. J Minim Invas Gyn. 2018;25(4):543-550. Accessed November 19, 2022.