Olympus to Distribute Terumo GLIDEWIRE®, Endoscopic Hydrophilic Coated Guidewire for Gastroenterological Endoscopy
Olympus Extends Its EndoTherapy Guidewire Line-Up to Provide the Proven, Preferred GLIDEWIRE® Endoscopic Hydrophilic Coated Guidewire
CENTER VALLEY, Pa., (December 15, 2015) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today its new 510(k) cleared EZDilate multi-stage endoscopic balloon dilator for improved precision and control in endoscopic balloon dilation procedures.
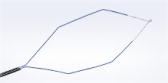
CENTER VALLEY, Pa., (December 15, 2015) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today its exclusive distributorship of the Terumo GLIDEWIRE® Endoscopic Hydrophilic Coated Guidewire for endoscopic treatment of the biliary and pancreatic systems. Beginning immediately, the Olympus EndoTherapy Sales team will distribute the Terumo GLIDEWIRE® Endoscopic Guidewire to U.S. customers.
The GLIDEWIRE® Endoscopic Guidewire is considered to be an exemplary guidewire for gastroenterological procedures and a well suited complement to the imaging and therapeutic device solutions currently offered by Olympus EndoTherapy. Through this partnership, Terumo Corporation and Olympus are able to expand access to the quality guidewire for physicians nationwide.
The GLIDEWIRE® Endoscopic Guidewire is used for biliary endoscopy, or endoscopic retrograde cholangiopancreatography (ERCP), a procedure that combines endoscopy and fluoroscopy (internal imaging to obtain real-time, moving X-ray images of internal structures). ERCP enables physicians to diagnose and treat problems in the gallbladder, bile ducts, pancreas and liver, such as gallstones, strictures, leaks resulting from trauma or surgery, and cancers. According to Truven Health Analytics data, more than 600,000 ERCP procedures were performed in the United States in 2013. During ERCP, guidewires are frequently used to obtain initial access to a target duct or confined space and then help advance bulkier devices to the desired location.
“I have used the GLIDEWIRE® for ERCP and for complex advanced endoscopic procedures for more than 20 years,” said Dr. Todd Baron, Director of Advanced Therapeutic Endoscopy at UNC. “I find it to be extremely useful for navigating difficult strictures, especially when other guidewires have failed. It is an indispensable tool for consistently succeeding in ERCP.”
The GLIDEWIRE® Endoscopic Guidewire is a complement to the VisiGlide guidewire line-up designed to provide successful pancreaticobiliary access. It provides precise ductal navigation, making it an excellent choice for pancreatic anatomy. It can be used for guiding and exchanging endoscopic accessories in the biliary system, including the common bile, cystic, pancreatic and right and left hepatic ducts. The GLIDEWIRE® Endoscopic Guidewire also is safe to use during sphincterotomy.
"By adding this trusted guidewire to our EndoTherapy line-up, we extend the guidewire options for complex ERCP procedures,” said Earl Adamy, Executive Director of Endotherapy Marketing at Olympus America Inc. “The GLIDEWIRE® Endoscopic Guidewire improves the quality of care by providing physicians access to complicated anatomy where they may not have had the benefit of access before.”
The GLIDEWIRE® Endoscopic Guidewire can help facilities address the three key requirements of healthcare reform in these ways:
- Increased Quality of Care: Precision of access, smooth advancement and easy tracking of instruments are benefits that in combination may provide quality-of-care advantages over other guidewires.
- Decreased Costs: Reliable access even in challenging cases while avoiding surgery to treat the problem.
- Enhanced Patient Satisfaction: Capability to protect against perforation and trauma can result in a reduced chance of patient complications.
For more information about the Terumo GLIDEWIRE® and the VisiGlide line-up, please contact Olympus customer service at 1-800-848-9024 Option 2 or visit medical.olympusamerica.com/products/ercp-guidewires.
# # #
About Terumo Medical Corporation
Founded in 1972 as a Terumo Corporation subsidiary, Terumo Medical Corporation (TMC) develops, manufactures, and markets a complete, solutions-based portfolio of high-quality medical devices used in a broad range of applications for numerous areas of the healthcare industry. TMC is comprised of two business divisions: Terumo Interventional Systems and Terumo Medical Products. The company places a premium on providing customers with world-class products, training and education programs that drive clear economic value, better clinical outcomes and improved Quality of Life for patients.
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare.
For more information visit Olympus at medical.olympusamerica.com.
i
Data for use in this study were supplied by Truven Health Analytics Inc., Ann Arbor, Michigan (“Truven Health”). Any analysis, interpretation or conclusion based on these data is solely that of the authors and Truven Health disclaims responsibility for any such analysis, interpretation or conclusion.
Dr. Baron is a contracted physician with Olympus.
GLIDEWIRE® Endoscopic Hydrophilic Coated Guidewire and GLIDEWIRE® Endoscopic Guidewire are registered trademarks of Terumo Corporation.