Olympus Announces New FDA Guidance and Labeling Changes Affecting PneumoLiner, the Only 510(k)-Cleared Containment Device
New FDA draft Guidance Now Mandates Surgeons Use Power Morcellation Only with an Approved Containment System for Gynecological Tissue

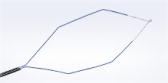
The ASC/Olympus PneumoLiner has undergone a labeling update, to allow for a wider range of patient candidates needing removal of non-malignant fibroids.
CENTER VALLEY, Pa., (March 13, 2020) – Olympus, a global technology leader in designing and delivering innovative solutions for medical procedures, among other core businesses, announced today that an updated guidance from the Food and Drug Administration (FDA) mandates that laparoscopic power morcellation in gynecological procedures be conducted with an approved containment device. When taken together with the updated labeling for 510(k) of the PneumoLiner, and an FDA safety update entitled “The FDA recommends performing contained morcellation in women when laparosopic morcellation is appropriate,” the agency is now offering a cohesive and succinct guidance for future use of laparoscopic power morcellation. PneumoLiner, made by Advanced Surgical Concepts (ASC) and exclusively distributed by Olympus, remains the only containment device 510(k)-cleared for use during power morcellation.
In its press release, the FDA states about the PneumoLiner: “While the device itself remains unchanged since its prior marketing authorization, today’s clearance updates the labeling for this device to better define the appropriate patient population for the safe and effective use of this device, including stating that the device should only be used in women who have fibroids if they are pre-menopausal and under 50 years old”.
The FDA safety communication update on power morcellation includes additional recommendations including:
- That health care providers use tissue containment systems when using laparoscopic power morcellators, and that they ensure the laparoscopic power morcellator and tissue containment system are compatible, legally marketed laparoscopic power morcellation containment systems intended to isolate and contain tissue that is considered benign.
- As demonstrated in testing, use of a containment system confines morcellated tissue within the containment system, which may prevent the peritoneal spread of cancerous tissue.
- Laparoscopic power morcellation (with a compatible containment system) should only be used in the appropriate patient population:
- women without uterine fibroids undergoing hysterectomy
- and women with fibroids, provided they are pre-menopausal and under age 50 with fibroids (with no suspicion of malignancy).
The leading gynecological laparoscopy association, AAGL, immediately endorsed the FDA’s guidance, with the following statement from its President, Jubilee Brown, MD: “The specification of age-related risk is consistent with scientific data and reflects clinical experience allowing power morcellation in low- risk patients when performed with a containment system compatible with the laparoscopic power morcellator. Utility of a contained tissue extraction system in a low- risk population appears to mitigate the risk of poor outcomes that may be associated with uncontained power morcellation.” The AAGL encouraged its members to submit electronic comments to the FDA’s Federal Register.
These modifications clarify power morcellation usage guidelines along with containment in appropriately selected patients. Further, the FDA clarifies the appropriate patient population by eliminating the ambiguous term “peri-menopausal” from the contraindications, and adds an easier-to-follow age restriction.
Those interested in the Olympus Contained Tissue Extraction system or PneumoLiner should contact their Olympus sales representatives for more information or visit https://medical.olympusamerica.com/products/contained-tissue-extraction-system.
# # #
About Olympus Medical Systems Group
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our 100-year history, Olympus has focused on being true to society and making people’s lives healthier, safer and more fulfilling.
Our Medical Business works with health care professionals to combine our innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing with their skills to deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance quality of life for patients. For more information, visit medical.olympusamerica.com.
About Advanced Surgical Concepts
Advanced Surgical Concepts (ASC) works closely with surgeons and specialist clinicians to conceive of new approaches to performing surgeries. In so doing, ASC designs and develops new medical devices in order to standardize the methods by which particular surgeries or diagnostic procedures will be performed. For more information, visit ASC at http://www.advancedsurgical.ie/.