Olympus Adds HANAROSTENT Esophagus Through the Scope Stent to its Expanding EndoTherapy Portfolio
New TTS Stent Allows Palliative Treatment of Esophageal Stricture and Trachea-Esophageal Fistula Caused by Malignant Tumors
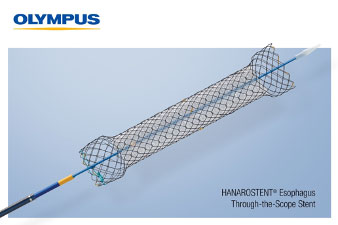
HANAROSTENT® Esophagus Through the Scope stents are 510(k) cleared devices made by M.I. Tech and now distributed exclusively through Olympus in the U.S for use in palliative treatment of esophageal stricture and/or trachea-esophageal fistula caused by malignant tumors.
CENTER VALLEY, Pa., (July 16, 2020) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today the 510(k) clearance and limited launch of HANAROSTENT® Esophagus TTS self-expanding metal stents (SEMS) made by M.I. Tech and now distributed exclusively through Olympus in the U.S.
“The HANAROSTENT Esophagus TTS expands our growing EndoTherapy portfolio and is critical to our mission of advancing the delivery of innovative medical technology,” said Kevin Mancini, Vice President for Endoscopy at Olympus America Inc. “We value our partnership with M.I. Tech and the opportunity to help physicians address quality of life issues in patients suffering from advanced gastrointestinal disease.”
The Through the Scope (TTS) esophageal stent, the newest addition to the Olympus self-expanding stent (SEMS) portfolio, helps to achieve luminal patency in a variety of clinical applications and is designed for use in palliative treatment of esophageal stricture and/or trachea-esophageal fistula caused by malignant tumors. The stent offers a fully covered or partially covered option.
Pre-loaded into the delivery system, the HANAROSTENT Esophagus TTS fits down a standard therapeutic gastroscope and is deployed for placement in the esophagus. The hook-cross Nitinol design, unique to HANAROSTENT products, enables optimal radial and axial force, allowing for the flexibility to conform to a patient’s anatomy and precisely target the stricture, ultimately providing better comfort to the patient, once placed.
Unlike over-the-wire esophageal stents, the new 10.5F HANAROSTENT Esophagus TTS allows for accurate and easy esophageal stenting under direct endoscopic visualization. Additional benefits of the new esophageal stent include:
- Minimized Use of Fluoroscopy: Direct visualization of the lumen allows physicians to place the stent with reduced dependence on fluoroscopy.
- Re-capturability: Ability to fully recapture stent up to a point-of-no-return, as well as a retrieval lasso for stent repositioning allows for safety and precision of placement for users of all experience levels.
- Long Delivery Length: The 180cm delivery length provides increased flexibility and application.
“We are pleased to expand capabilities of U.S. physicians in the treatment of esophageal stricture and trachea-esophageal fistula through the launch of HANAROSTENT Esophagus TTS,” said Jin-Hyung Park, CEO at M.I.Tech Co. Ltd. “Our trusted partnership with Olympus has allowed us to focus our attention on thoughtful innovation focused on improving patient care and quality of life benefits to patients all over the world.”
For more information on the FDA Clearance and launch of HANAROSTENT Esophagus TTS, please contact Olympus customer service at 1-800-848-9024 or contact your local representative.
# # #
About Olympus Medical Systems Group
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our 100-year history, Olympus has focused on being true to society and making people’s lives healthier, safer and more fulfilling.
Our Medical Business works with health care professionals to combine our innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing with their skills to deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance quality of life for patients. For more information, visit medical.olympusamerica.com.
About M.I. Tech
M.I. Tech Co., Ltd. develops, manufactures, and provides self-expandable metallic stents as a leading company in the field of interventional medicine. Since 1991 with its headquarters in Pyeongtaek, South Korea, the company has provided and upheld worldwide a superior standard of less invasive palliative treatment. By utilizing years of accumulated know-how and maintaining the global standard, our full range of gastrointestinal and airway stents are being manufactured under the strict quality management system, ISO 13485:2003, to satisfy the quality demands from our customers and to fulfill our mission, Create the Health. For more information visit M.I. Tech at www.mitech.co.kr